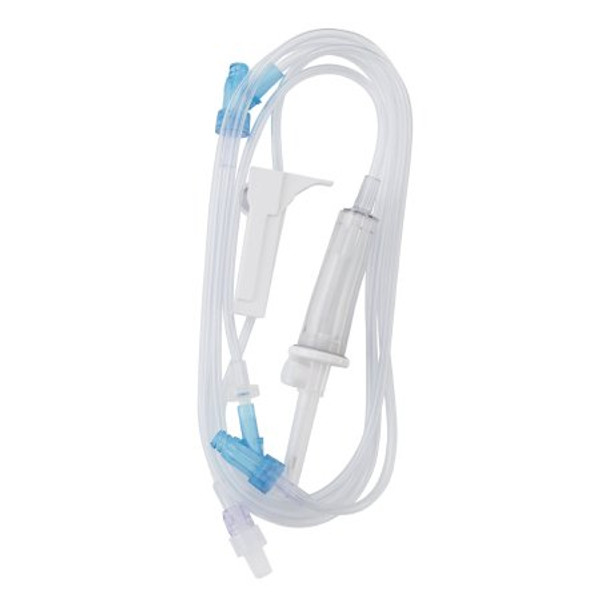
Product Overview
| Manufacturer # | 352639 |
| Brand | SafeDay™ |
| Manufacturer | B. Braun |
| Country of Origin | Unknown |
| Administration Method | Gravity |
| Application | Primary IV Administration Set |
| Back Check Valve | Back Check Valve |
| Connection Type | Spin-Lock® Connector |
| DEHP Indicator | DEHP Tubing |
| Drip Rate | 15 Drops / mL Drip Rate |
| Filter | Without Filter |
| Flow Control Type | Roller Clamp / Slide Clamp |
| Flow Regulator | Without Flow Regulator |
| Injection Port Type Grouping | Needle-Free Port |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | Y |
| Lot_Tracking_Flag | N |
| Number of Ports | 2 Ports |
| On_Allocation | N |
| Port Type | SafeDay™ Valve Port |
| Priming Volume | 17.2 mL Priming Volume |
| Product Dating | McKesson Acceptable Dating: we will ship >= 90 days |
| Spike Type | Universal Spike |
| Sterility | Sterile |
| Supplier_ID | 487832 |
| Tubing Length | 84 Inch Tubing |
| Tubing Length Range | 73 to 96 Inches |
| Type | Solution |
| UNSPSC Code | 42221609 |
QTY CASE of 50

 Help
Help